UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): May 12, 2020
KRYSTAL BIOTECH, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 001-38210 | 82-1080209 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(IRS Employer Identification Number) |
2100 Wharton Street, Suite 701
Pittsburgh, Pennsylvania 15203
(Address of principal executive offices, including Zip Code)
Registrant’s telephone number, including area code: (412) 586-5830
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered | ||
| Common Stock | KRYS | Nasdaq |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
| Item 8.01 | Other Events. |
On May 12, 2020, Krystal Biotech, Inc., a Delaware corporation (the “Company”), presented a poster on the in vitro pharmacology of KB407, an HSV-1-based gene therapy vector, for the treatment of cystic fibrosis at the American Society of Gene & Cell Therapy Annual Meeting. A copy of the Company’s poster is attached as Exhibit 99.1 hereto and incorporated by reference herein and is also available at the Company’s website located at www.krystalbio.com/select-scientific-publications.
Any statements in this Current Report on Form 8-K about future expectations, plans and prospects for Krystal Biotech, Inc., including but not limited to statements about the development of Krystal’s product candidates, such as plans for the design, conduct and timelines of ongoing clinical trials of beremagene geperpavec (“B-VEC”), KB105 and KB407; the clinical utility of B-VEC, KB105 and KB407, and Krystal’s plans for filing of regulatory approvals and efforts to bring B-VEC, KB105 and KB407 to market; the market opportunity for and the potential market acceptance of B-VEC, KB105 and KB407; plans to pursue research and development of other product candidates; the sufficiency of Krystal’s existing cash resources; the unanticipated impact of COVID-19 on Krystal’s business operations, pre-clinical activities and clinical trials; and other statements containing the words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “likely,” “will,” “would,” “could,” “should,” “continue,” and similar expressions, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including: the uncertainties inherent in the initiation and conduct of clinical trials, availability and timing of data from clinical trials, whether results of early clinical trials or trials will be indicative of the results of ongoing or future trials, uncertainties associated with regulatory review of clinical trials and applications for marketing approvals, the availability or commercial potential of product candidates including B-VEC, KB105 and KB407, the sufficiency of cash resources and need for additional financing and such other important factors as are set forth under the caption “Risk Factors” in Krystal’s annual and quarterly reports on file with the U.S. Securities and Exchange Commission. In addition, the forward-looking statements included in this press release represent Krystal’s views as of the date of this release. Krystal anticipates that subsequent events and developments will cause its views to change. However, while Krystal may elect to update these forward-looking statements at some point in the future, it specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing Krystal’s views as of any date subsequent to the date of this release.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits.
| Exhibit No. |
Description | |
| 99.1 |
In Vitro Pharmacology of KB407, An HSV-1-Based Gene Therapy Vector, for the Treatment of Cystic Fibrosis Poster, dated May 12, 2020 | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Date: May 13, 2020 | KRYSTAL BIOTECH, INC. | |||||
| By: | /s/ Krish S. Krishnan | |||||
| Name: | Krish S. Krishnan | |||||
| Title: | President and Chief Executive Officer | |||||
Exhibit 99.1
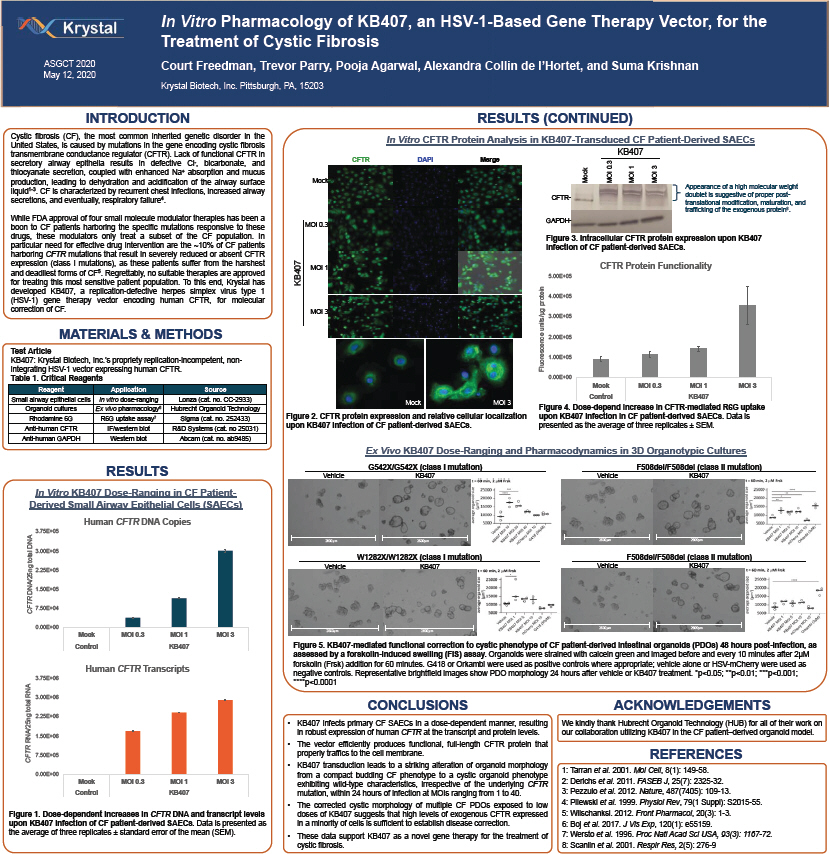
In Vitro Pharmacology of KB407, an HSV-1-Based Gene Therapy Vector, for the Treatment of Cystic Fibrosis ASGCT 2020 Court Freedman, Trevor Parry, Pooja Agarwal, Alexandra Collin de l’Hortet, and Suma Krishnan May 12, 2020 Krystal Biotech, Inc. Pittsburgh, PA, 15203 INTRODUCTION RESULTS (CONTINUED) Cystic fibrosis (CF), the most common inherited genetic disorder in the In Vitro CFTR Protein Analysis in KB407-Transduced CF Patient-Derived SAECs United States, is caused by mutations in the gene encoding cystic fibrosis transmembrane conductance regulator (CFTR). Lack of functional CFTR in KB407 CFTR DAPI Merge secretory airway epithelia results in defective Cl-, bicarbonate, and 3 . 0 13 thiocyanate secretion, coupled with enhanced Na+ absorption and mucus production, leading to dehydration and acidification of the airway surface Mock Mock MOI MOI MOI liquid1-3. CF is characterized by recurrent chest infections, increased airway Appearance of a high molecular weight CFTR- doublet is suggestive of proper post- secretions, and eventually, respiratory failure4. translational modification, maturation, and trafficking of the exogenous protein8. While FDA approval of four small molecule modulator therapies has been a GAPDH-boon to CF patients harboring the specific mutations responsive to these MOI 0.3 drugs, these modulators only treat a subset of the CF population. In Figure 3. Intracellular CFTR protein expression upon KB407 particular need for effective drug intervention are the ~10% of CF patients infection of CF patient-derived SAECs. harboring CFTR mutations that result in severely reduced or absent CFTR expression (class I mutations), as these patients suffer from the harshest CFTR Protein Functionality MOI 1 and deadliest forms of CF5. Regrettably, no suitable therapies are approved 5.00E+05 for treating this most sensitive patient population. To this end, Krystal has KB407 developed KB407, a replication-defective herpes simplex virus type 1 (HSV-1) gene therapy vector encoding human CFTR, for molecular protein 4.00E+05 correction of CF. MOI 3 units/µg 3.00E+05 MATERIALS & METHODS 2.00E+05 Test Article KB407: Krystal Biotech, Inc.’s propriety replication-incompetent, non- Fluorescence 1.00E+05 integrating HSV-1 vector expressing human CFTR. Table 1. Critical Reagents 0.00E+00 Mock MOI 0.3 MOI 1 MOI 3 Reagent Application Source Small airway epithelial cells In vitro dose-ranging Lonza (cat. no. CC-2933) Control KB407 Mock MOI 3 Organoid cultures Ex vivo pharmacology6 Hubrecht Organoid Technology Figure 4. Dose-depend increase in CFTR-mediated R6G uptake Rhodamine 6G R6G uptake assay7 Sigma (cat. no. 252433) Figure 2. CFTR protein expression and relative cellular localization upon KB407 infection in CF patient-derived SAECs. Data is Anti-human CFTR IF/western blot R&D Systems (cat. no 25031) upon KB407 infection of CF patient-derived SAECs. presented as the average of three replicates ± SEM. Anti-human GAPDH Western blot Abcam (cat. no. ab9485) Ex Vivo KB407 Dose-Ranging and Pharmacodynamics in 3D Organotypic Cultures RESULTS G542X/G542X (class I mutation) F508del/F508del (class II mutation) Vehicle KB407 Vehicle KB407 In Vitro KB407 Dose-Ranging in CF Patient-Derived Small Airway Epithelial Cells (SAECs) Human CFTR DNA Copies 3.75E+05 DNA 2600µm 2600µm 2600µm 2600µm 3.00E+05 total W1282X/W1282X (class I mutation) F508del/F508del (class II mutation) Vehicle KB407 Vehicle KB407 2.25E+05 DNA/25ng 1.50E+05 CFTR 7.50E+04 0.00E+00 2600µm 2600µm 2600µm 2600µm Mock MOI 0.3 MOI 1 MOI 3 Control KB407 Figure 5. KB407-mediated functional correction to cystic phenotype of CF patient-derived intestinal organoids (PDOs) 48 hours post-infection, as assessed by a forskolin-induced swelling (FIS) assay. Organoids were strained with calcein green and imaged before and every 10 minutes after 2µM forskolin (Frsk) addition for 60 minutes. G418 or Orkambi were used as positive controls where appropriate; vehicle alone or HSV-m Cherry were used as Human CFTR Transcripts negative controls. Representative brightfield images show PDO morphology 24 hours after vehicle or KB407 treatment. *p<0.05; **p<0.01; ***p<0.001; 3.75E+06 ****p<0.0001 RNA 3.00E+06 total CONCLUSIONS ACKNOWLEDGEMENTS 2.25E+06 KB407 infects primary CF SAECs in a dose-dependent manner, resulting We kindly thank Hubrecht Organoid Technology (HUB) for all of their work on in robust expression of human CFTR at the transcript and protein levels. our collaboration utilizing KB407 in the CF patient–derived organoid model. RNA/25ng 1.50E+06 The vector efficiently produces functional, full-length CFTR protein that 100µm properly traffics to the cell membrane. CFTR 7.50E+05 REFERENCES KB407 transduction leads to a striking alteration of organoid morphology 1: Tarran et al. 2001. Mol Cell, 8(1): 149-58. 0.00E+00 from a compact budding CF phenotype to a cystic organoid phenotype , 25(7): 2325- . Mock MOI 0.3 MOI 1 MOI 3 exhibiting wild-type characteristics, irrespective of the underlying CFTR 2: Derichs et al. 2011. FASEB J 32 Control KB407 mutation, within 24 hours of infection at MOIs ranging from 1 to 40. 3: Pezzulo et al. 2012. Nature, 487(7405): 109-13. The corrected cystic morphology of multiple CF PDOs exposed to low 4: Pilewski et al. 1999. Physiol Rev, 79(1 Suppl): S2015-55. Figure 1. Dose-dependent increases in CFTR DNA and transcript levels doses of KB407 suggests that high levels of exogenous CFTR expressed 5: Wilschanksi. 2012. Front Pharmacol, 20(3): 1-3. upon KB407 infection of CF patient-derived SAECs. Data is presented as in a minority of cells is sufficient to establish disease correction. 6: Boj et al. 2017. J Vis Exp, 120(1): e55159. the average of three replicates ± standard error of the mean (SEM). These data support KB407 as a novel gene therapy for the treatment of 7: Wersto et al. 1996. Proc Natl Acad Sci USA, 93(3): 1167-72. cystic fibrosis. 8: Scanlin et al. 2001. Respir Res, 2(5): 276-9
